15 All atoms have same number protons. Atoms of a given element.

Solved 44 All Atoms Of A Given Element Have The Same A Chegg Com
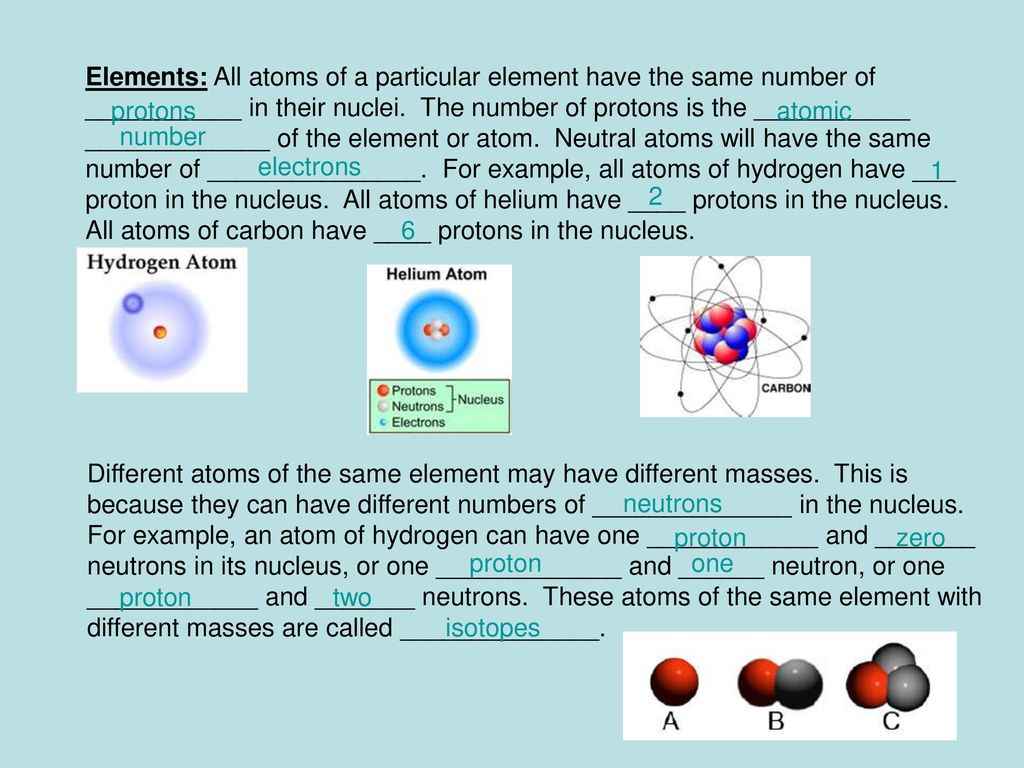
Daltons Atomic Theory 1804 All matter is composed of extremely small particles called atoms.
. Atoms combine in simple whole- number ratios to form compounds. 1 See answer Advertisement. The cu63 and isotope of cu65 cu63 cu atomic numper is 29 its taken the proton.
All atoms of a given element have the samea. C The isotopes of iodine will always have the same number of neutrons but the protons can vary. All of the above are correct.
A The isotopes of iodine have between 55 and 91 protons. Isotopes have the same atomic number but different atomic masses. All atoms of the same element have the same ____.
What does 84 repersent in the name krypton-84. The nuclei of all atoms of a given element always have the same number of _____. Each element is composed of tiny indestructible particles called atoms.
Atoms of an element share that elements chemical and physical properties such as boiling point melting point and stability. Protonsneutrons in their nuclei. The masses amu and abundances ofthe isotopes are given in.
Which one of the following is the minor product expected from the SN1 reaction given. Question 5 5 5 points All atoms of a given element have the same __________. All atoms of the same element have.
All atoms of an element have the same number of protons. Chalcogen noble gas halogen. 1Each element is composed of tiny indestructible particles called atoms.
D All atoms of a given element have the same 1 point C number of neutrons E density B number of protons D number of electrons and neutrons 2 Of the choices below which one is not an ionic compound. Number of neutrons Question 6 5 5 points Oxygen gas is considered a _____. View the full answer.
1 point A PCI BMoCl CRbCl D PbC12 E NaCł. Every atom of carbon must contain _____ but some contain six. Number of electrons c.
Answer and Explanation. Experimental evidence has shown that in most cases all atoms of a given element do not have identical masses. All atoms of an element have the same number of protons and every element has a different numbee of protons in its atoms.
Atoms of the same element that have a different number of neutrons are called isotopes. B An atom of iodine can have between 55 and 91 neutrons. E equal number of neutrons.
All atoms are neutral with the number of protons equaling the. Number of protons d. An element is the simplest form of matter and cannot be broken.
Asked Sep 1 2021 in Nursing by JagerMeister. Question 7 5 5 points What is the name for iron. Asked Sep 1 2021 in Nursing by dtn1267.
They cant be created or destroyed. The nucleus can. Number of neutrons c.
All neutral atoms of an element have the same a. Isotopes of the same element have different. General Organic and Biological Chemistry 5th Edition Edit edition Solutions for Chapter 3 Problem 98EP.
Because the number of protons determines Z the atomic number which determines the identity of the atom. All atoms of a given element have the same number of 1. Atoms combine in simple whole-number ratios to form compounds.
D different numbers of protons. Unequal number of hydrogen atoms are attached and the carbon atoms attach to each other with a double bond. All atoms of a given element have the same mass and other properties that distinguish them from the atoms of other elements.
The number of protons in an element is a primary identifier known as its atomic number. If you want to know the number of neutrons in. E protons neutrons.
All elements of the same element have the same. Number of electrons and neutrons mass number of protons. For a given element the number of postively charged massive nuclear particles the number of protons is fixed.
Each carbon has two attached hydrogen atoms. This is because atoms of the same element may have different numbers of _____ 2. 3 Calculate the number of electrons for the following species.
The atomic mass depends on the number of protons and neutrons massive nuclear particles. The properties of an element are related to the. Number of protons d.
Subsequently question is are all atoms of an element identical. 2All atoms of a given element have the same mass and other properties that distinguish them from the atoms of other elements. A protons electrons B neutrons protons C protons neutrons D electrons protons E neutrons electrons.
B equal number of protons. For example all helium atoms have two protons and no other elements have atoms with two protons Advertisement Still have questions. Isotopes are atoms that have the same number of __________ but differing number of __________.
Question 5 5 5 points all atoms of a given element. Medium Solution Verified by Toppr Correct option is B Atoms of the same element have the same atomic number so the number of protons and electrons are same. Could somebody help me with this.
A different numbers of electrons. Who discoered the neutron and what year. The main problem with a solar system model of the atom is that.
1 point 54 4 Give the formula for. D The isotopes of iodine have between 108 and 144 neutrons but the number of protons will not vary. Atoms of the same element with different numbers of neutrons are called _____ Isotopes.
C different numbers of neutrons. Find more answers Ask your question New questions in Science. All atoms of a given element have the same A mass B number of protons C number of neutrons D number of electrons and neutrons E density 16.
Element X has three naturally occurring isotopes.

Solved D All Atoms Of A Given Element Have The Same 1 Chegg Com

Chapter 11 Introduction To Atoms Ppt Download

Solved 6 Isotopes Of A Given Element Have The Same Number Chegg Com

Isotopes All Atoms Of An Element Have The Same Number Of Protons However They Can Have A Different Number Of Neutrons What Do We Call An Atom When They Ppt Video
0 Comments